Sulfenyl Chlorides: An Alternative Monomer Feedstock from Elemental Sulfur for Polymer Synthesis
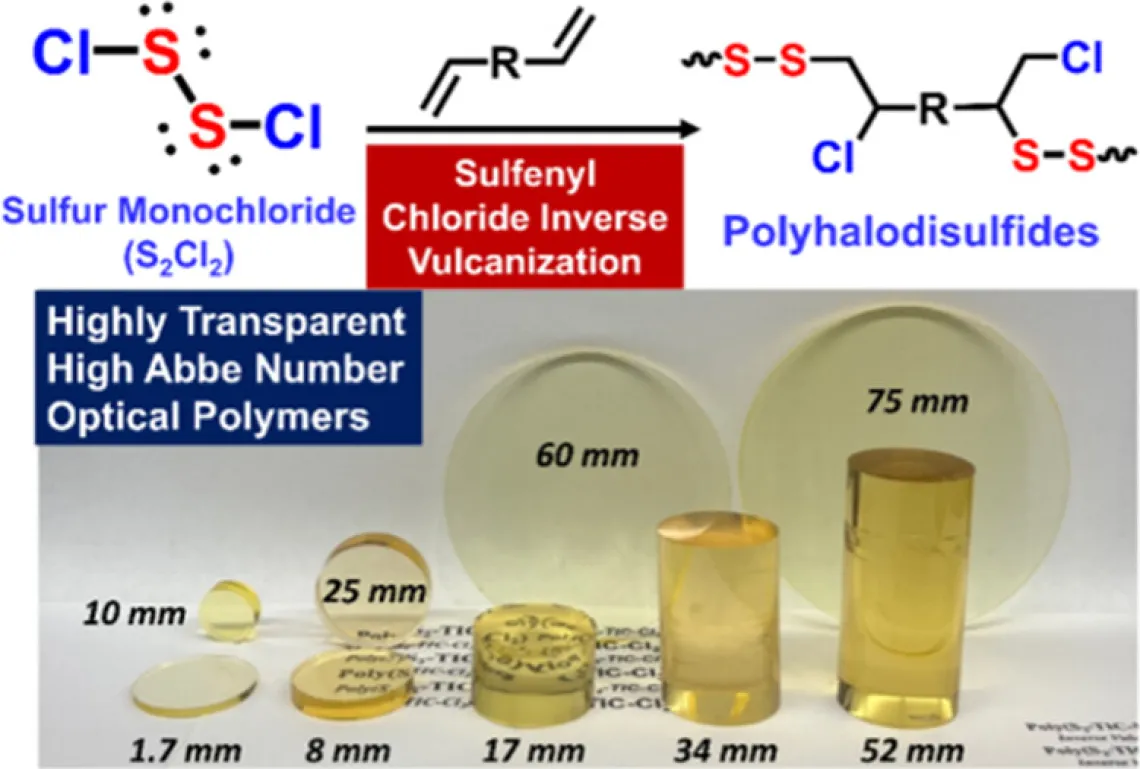
A polymerization methodology is reported using sulfur monochloride (S2Cl2) as an alternative feedstock for polymeric materials. S2Cl2 is an inexpensive petrochemical derived from elemental sulfur (S8) but has numerous advantages as a reactive monomer for polymerization vs S8. This new process, termed sulfenyl chloride inverse vulcanization, exploits the high reactivity and miscibility of S2Cl2 with a broad range of allylic monomers to prepare soluble, high molar-mass linear polymers, segmented block copolymers, and crosslinked thermosets with greater synthetic precision than achieved using classical inverse vulcanization. This step-growth addition polymerization also allows for preparation of a new class of thiol-free, inexpensive, highly optically transparent thermosets (α = 0.045 cm–1 at 1310 nm), which exhibit among the best optical transparency and low birefringence relative to commodity optical polymers, while possessing a higher refractive index (n > 1.6) in the visible and near-infrared spectra. The fabrication of large-sized optical components is also demonstrated.
