Synthesis of Metallopolymers via Atom Transfer Radical Polymerization from a [2Fe-2S] Metalloinitiator: Molecular Weight Effects on Electrocatalytic Hydrogen Production
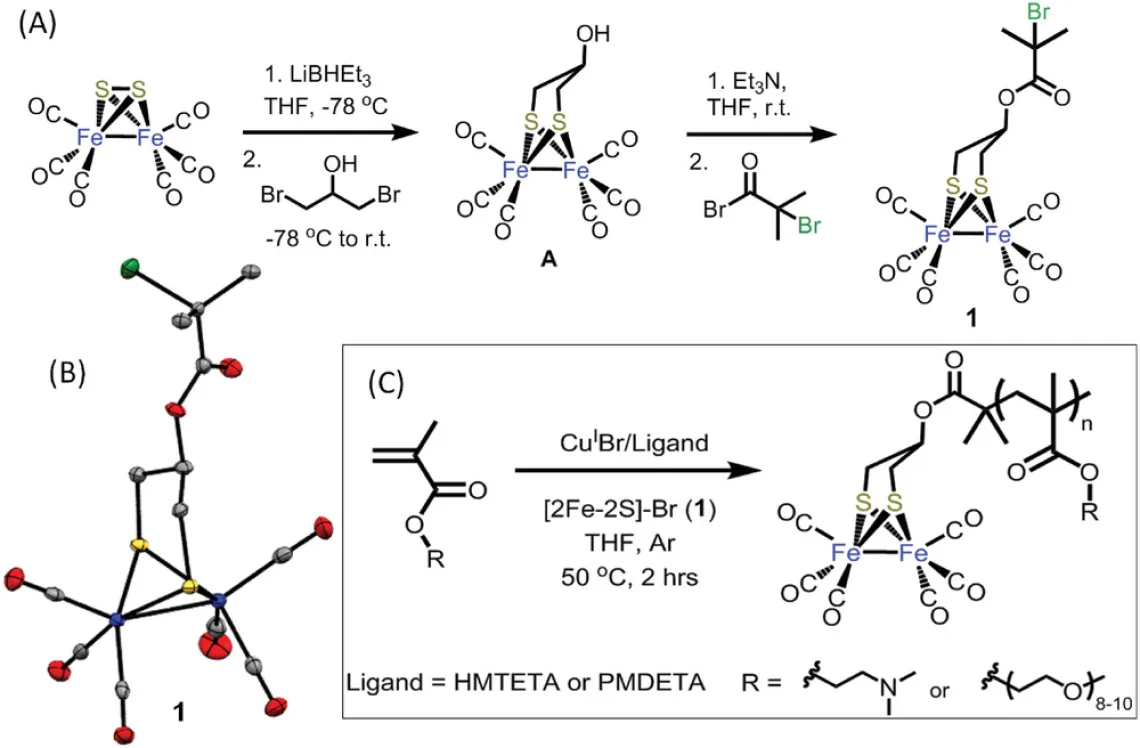
Abstract: Small molecule biomimetics inspired by the active site of the [FeFe]-hydrogenase enzymes have shown promising electrocatalytic activity for hydrogen (H2) generation. However, most of the active-site mimics based on [2Fe-2S] clusters are not water-soluble which limits the use of these electrocatalysts to organic media. Polymer-supported [2Fe-2S] systems, in particular, single-site metallopolymer catalysts, have shown drastic improvements for electrocatalytic H2 generation in aqueous milieu. [2Fe-2S] complexes functionalized within well-defined macromolecular supports via covalent bonding have demonstrated water solubility, enhanced site-isolation, and improved chemical stability during catalysis. In this report, the synthesis of a new propanedithiolate (pdt)-[2Fe-2S] complex bearing a single α-bromoester moiety for use in atom transfer radical polymerization (ATRP) is demonstrated as a novel metalloinitiator to prepare water-soluble poly(2-dimethylaminoethyl methacrylate) grafted (PDMAEMA-g-[2Fe-2S]) metallopolymers. Using this approach, metallopolymers with controllable molecular weights (Mn = 5–40 kg mol−1) and low dispersity (Đ, Mw/Mn = 1.09–1.36) are prepared, which allows for the first time observation of the effect of the metallopolymers' chain length on the electrocatalytic activity. The ability to control the composition and molecular weight of these metallopolymers enables macromolecular engineering via ATRP of these materials to determine optimal structural features of metallopolymer catalysts for H2 production.
